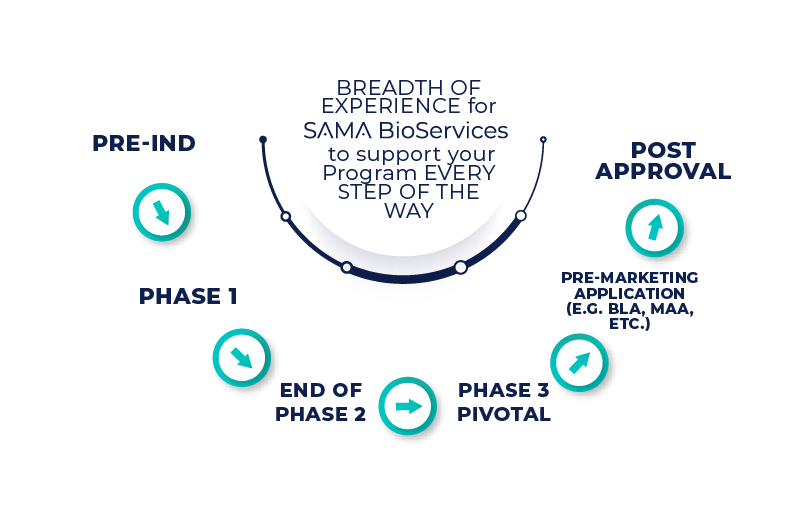
offers expertise in areas of CMC Drug Product Development/Manufacturing, CDMO Management/Oversight, PIP, CMC Regulatory Authoring & Advise, Quality Assurance, CMC Supply Chain Management, Project Management. Our offer covers a wide range of experience, focused on quality-based drug products process development, manufacturing, validation and troubleshooting, stability strategy and evaluation.
Our CMC Drug Product solutions include preparation and review of regulatory submissions, risk-based strategies for product development, PAI readiness, oversight of Contract Services, supply chain management, project management, and the design, implementation of GMP Systems.